X4 Pharmaceuticals Presents New Clinical Data at ASH 2021 Further Supporting Potential of Mavorixafor + Ibrutinib to Treat Patients with Double-Mutation Waldenström’s Macroglobulinemia
- CXCR4 antagonism proof of concept established, with 100% overall response rate to combination treatment in frontline and refractory patients -
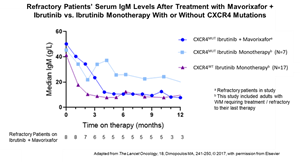
- Median Serum IgM reductions in refractory CXCR4MUT patients on combination therapy similar to those seen in CXCR4WT patients on ibrutinib monotherapy -
- Study enrollment ongoing at highest dose of mavorixafor; longer-term response data, dose selection, and regulatory updates expected in 2022 -
“We continue to be excited by the data from our ongoing Phase 1b clinical trial, with 100% of evaluable patients achieving an overall response (OR) to date,” said
What’s New in This Poster?
- As of
October 12, 2021 , 16 patients were enrolled in the Phase 1b clinical trial, with 14 being evaluable as of the data cut, all at low- (200 mg) and mid-level (400 mg) dosing of mavorixafor; median duration of treatment was 272.5 days (range 33-435 days); 12 patients remain on study.
For all patients on study (both frontline and refractory):
- The median level of serum IgM reduced from 47.2 g/L (N=14) at baseline to 7.73 g/L (N=3) after 12 months of treatment.
- In 10 patients evaluable for response, the overall response rate was 100% (ORR = >25% reduction in serum IgM), with four achieving a Major Response (>50% reduction in serum IgM), including one Very Good Partial Response (VGPR) of >90% reduction in serum IgM.
- The median level of hemoglobin approached normal, increasing by approximately 38 g/L from baseline (N=14) to month 12 (N=3).
- Overall, the combination of mavorixafor and ibrutinib was tolerated with a manageable safety profile at the low- and mid-level dosing of mavorixafor; dose escalation to 600 mg mavorixafor is ongoing.
For patients with refractory disease:
- Median decreases in serum IgM from 50.2 g/L at baseline (N=8) to 7.7 g/L at 12 months (N=3) were similar to decreases seen in a previous study of ibrutinib monotherapy in Waldenström’s patients without CXCR4 mutations.
- An ORR of 100% (N=7), with two achieving a Major Response, including one VGPR, was seen.
- Median time-on-treatment for patients achieving a Major Response was 51.7 weeks (N=3); median time on treatment for patients achieving Minor Responses (MR = 25% to <50% reduction in serum IgM) was 30.1 weeks (N=4).
- The median level of hemoglobin approached normal, increasing by approximately 32 g/L from baseline (N=18) to month 12 (N=1).
About Waldenström’s Macroglobulinemia and Associated CXCR4 Mutations
Waldenström’s macroglobulinemia is a rare B-cell lymphoproliferative disorder characterized by increased immunoglobulin M (IgM). Greater than 90% of patients with Waldenström’s have acquired mutations in the MYD88 gene, with a subset (30%–40%) also having mutations in chemokine receptor CXCR4. The presence of the CXCR4 mutation is associated with greater cancer burden, higher serum IgM levels, and increased risk of developing a serious emergent condition called symptomatic hyperviscosity syndrome. Importantly, the presence of CXCR4 mutations has been shown to negatively impact patients’ response to ibrutinib (a BTK inhibitor), as manifested by delayed response, inferior depth of response, and/or shorter progression-free survival.
About Mavorixafor and the Phase1b Clinical Trial in Waldenström’s Macroglobulinemia
Mavorixafor is an oral small-molecule antagonist of the CXCR4 receptor with a demonstrated ability to inhibit binding of its ligand, CXCL12; this inhibition of CXCR4 has been shown to sensitize Waldenström’s cells with both MYD88 and CXCR4 mutations to BTK antagonists, such as ibrutinib. The ongoing Phase 1b, open-label, multicenter, single-arm study (NCT04274738) examines intra-patient dose escalation, safety, pharmacokinetics, and pharmacodynamics of mavorixafor in combination with ibrutinib in up to 18 patients aged 18 years or older with a diagnosis of Waldenström’s macroglobulinemia and confirmed MYD88 and CXCR4 genetic mutations. In the study, all cohorts are dosed with oral, once-daily doses of ibrutinib 420 mg. In Cohorts A and B, patients are initiated on oral, once-daily mavorixafor 200 mg (low dose) and are escalated to 400 mg mavorixafor (mid-dose) after 28 days if no dose-limiting toxicities are observed in five of six participants. Patients in Cohort C are initiated at 400 mg mavorixafor. Participants are escalated to 600 mg mavorixafor (high dose) after 400 mg is deemed tolerable. All patients are followed for adverse events and change from baseline in serum IgM and hemoglobin, pharmacokinetics, and pharmacodynamic markers, including peripheral white blood cell counts (WBCs) and clinical response.
About
Forward Looking Statements:
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. These statements may be identified by the words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target,” or other similar terms or expressions that concern X4's expectations, strategy, plans, or intentions. Forward-looking statements include, without limitation, statements regarding the clinical development and therapeutic potential of mavorixafor and the advancement of X4’s pipeline. Any forward-looking statements in this press release are based on management's current expectations and beliefs. Actual events or results may differ materially from those expressed or implied by any forward-looking statements contained herein, including, without limitation, uncertainties inherent in the initiation and completion of preclinical studies and clinical trials and clinical development; the risk that trials and studies may be delayed, including, but not limited to, as a result of the effects of the ongoing COVID-19 pandemic or delayed patient enrollment, and may not have satisfactory outcomes; the risk that the outcomes of preclinical studies or earlier clinical trials will not be predictive of later clinical trial results; the potential adverse effects arising from the testing or use of mavorixafor or other product candidates; risks related to X4’s ability to raise additional capital and other risks and uncertainties, including those described in the section entitled “Risk Factors” in X4’s Quarterly Report on Form 10-Q filed with the
Contacts:
VP, Investor Relations & Corporate Communications
glenn.schulman@x4pharma.com
(203) 494-7411
Managing Director,
daniel@lifesciadvisors.com
(617) 430-7576
Senior Account Executive,
mroucomolina@lifescicomms.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/4e5fbf54-18ea-4901-a6e5-154c2a996e75

Source: X4 Pharmaceuticals