UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
| FORM | | ||||
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Data of earliest event reported): August 5, 2022
| (Exact name of registrant as specified in its charter) | ||||||||
| | ||||||||||||||
| (State or other jurisdiction of incorporation) | (Commission File Number) | (IRS Employer Identification No.) | ||||||||||||
| (Address of principal executive offices) | (Zip Code) | ||||||||||
(857 ) 529-8300
(Registrant’s telephone number, including area code)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||||||||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (240.12b-2 of this chapter). Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act ☒
| Item 7.01 | Regulation FD Disclosure. | ||||
X4 Pharmaceuticals, Inc. (the “Company”) has updated its corporate presentation to be used from time to time when management of the Company presents at various industry and other conferences. On August 5, 2022, the Company updated its corporate presentation, which is available on the “Events and Presentation” section of the Company’s investor relations website at http://investors.x4pharma.com. A copy of the updated corporate presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information in this Item 7.01 and in the corporate presentation attached as Exhibit 99.1 to this Current Report on Form 8-K shall not be deemed to be “filed” for purposes of Section 18 of the Securities Act of 1934, as amended, or otherwise subject to the liabilities of that Section, nor shall they be deemed incorporated by reference into any registration statement or other filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
The corporate presentation attached as Exhibit 99.1 to this Current Report on Form 8-K includes “safe harbor” language pursuant to the Private Securities Litigation Reform Act of 1995, as amended, indicating that certain statements contained therein are “forward-looking” rather than historical.
The Company undertakes no duty or obligation to update or revise the information contained in this Current Report on Form 8-K, although it may do so from time to time if its management believes it is appropriate. Any such updating may be made through the filing of other reports or documents with the Securities and Exchange Commission, through press releases or through other public disclosures.
| Item 9.01 | Financial Statements and Exhibits. | |||||||
| Exhibit No. | Description | |||||||
| 99.1 | ||||||||
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | |||||||
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934 the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| X4 PHARMACEUTICALS, INC. | |||||||||||
| Date: August 5, 2022 | By: | /s/ Derek Meisner | |||||||||
| Derek Meisner | |||||||||||
| Chief Legal Officer | |||||||||||

Corporate Overview August 2022

2 Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. These statements may be identified by the words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target,” or other similar terms or expressions that concern X4's expectations, strategy, plans, or intentions. Forward-looking statements include, without limitation, statements regarding the clinical development and therapeutic potential of mavorixafor for the treatment of WHIM syndrome, chronic and other neutropenias, and of X4’s other product candidates; X4’s possible exploration of additional opportunities for mavorixafor; the expected duration of patent protection; the expected availability, content and timing of clinical data from X4’s ongoing clinical trials of mavorixafor; anticipated regulatory filings; clinical trial design; patient prevalence; market opportunities; and X4’s cash runway and ability to satisfy covenants in agreements with third parties. Any forward-looking statements in this presentation are based on management's current expectations and beliefs. Actual events or results may differ materially from those expressed or implied by any forward-looking statements contained herein, including, without limitation, uncertainties inherent in the initiation and completion of preclinical studies and clinical trials and clinical development; the risk that trials and studies may be delayed, including, but not limited to, as a result of the effects of the ongoing COVID-19 pandemic, and may not have satisfactory outcomes; the risk that the outcomes of preclinical studies or earlier clinical trials will not be predictive of later clinical trial results; the risk that initial or interim results from a clinical trial may not be predictive of the final results of the trial or the results of future trials; the potential adverse effects arising from the testing or use of mavorixafor or other product candidates; the risk that patient prevalence, market or opportunity estimates may be inaccurate; risks related to X4’s ability to raise additional capital; risks related to the substantial doubt about X4’s ability to continue as a going concern; and other risks and uncertainties, including those described in the section entitled “Risk Factors” in X4’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (SEC) on August 4, 2022, and in other filings X4 makes with the SEC from time to time. X4 undertakes no obligation to update the information contained in this presentation to reflect new events or circumstances, except as required by law. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third- party sources and X4’s own internal estimates and research. While X4 believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy, or completeness of, any information obtained from third-party sources. Finally, while X4 believes its own internal research is reliable, such research has not been verified or validated by any independent source.

3 A Unique Investment Opportunity with Significant Near-Term Milestones Unparalleled expertise in CXCR4 biology Antagonizing the CXCR4/CXCL12 axis proven to increase the expansion, maturation and mobilization of white blood cells, including neutrophils Mavorixafor – a late-stage clinical CXCR4 antagonist candidate Designed to be a once-daily oral therapy enabling the mobilization of immune cells from the bone marrow to the blood to improve immune system function Key mavorixafor upcoming clinical milestones expected: • Results from Phase 1b trial in chronic neutropenia in late September 2022 • Results from global, pivotal Phase 3 trial in WHIM syndrome in 4Q 2022 • U.S. NDA filing expected in WHIM in early 2H 2023 Strong balance sheet, with cash runway expected to fund operations into 3Q 2023 July 2022 fundraising, re-prioritization of resources, and other measures projected to extend runway though key upcoming milestones Sharp focus on chronic neutropenic disorders Bringing innovative treatments to >5,000 estimated patients with rare diseases of the immune system and high unmet needs

4 Seasoned Executive Leadership Team Continues to Execute on Mission PAULA RAGAN, Ph.D. President & CEO ADAM MOSTAFA Chief Financial Officer MARY DIBIASE, Ph.D. Chief Operating Officer DIEGO CADAVID, M.D. Chief Medical Officer ART TAVERAS, Ph.D. Chief Scientific Officer

5 The Dysregulated Immune System: Immunodeficiencies Chronic Immunosuppression • Life-long severe and/or life-threatening infections • Reduced/no response to vaccines • Increased cancer risk • High morbidity (bronchiectasis, hearing loss) and life-threatening sepsis Caused By • Low white blood cell counts (cytopenias) and/or • Dysregulated or dysfunctional immune cells Treated with Injectables • Antibiotics for acute infections • G-CSF for those with severe neutropenia • IVIG for those with hypogammaglobulinemia (prevention) • Stem cell transplant in rare/severe cases Unmet need: Oral treatment that corrects and regulates a range of immune system deficiencies Chronic Neutropenias2 > 5,000 U.S. patients (severe and moderate) WHIM Syndrome3 > 1,000 U.S. patients Common Variable Immunodeficiency (CVID)1 > 10,000 U.S. patients Immunodeficiencies (U.S. Prevalence) 1. Odnoletkova et al Orphanet Journal of Rare Diseases v13, 201 (2018). 2. US estimate for severe plus 10% penetrance for moderate and prevalence from Andersen et al. J Intern Med. 2016 Jun;279(6):566-75. 3. Company market research. Qessential market research, 2019 and IPM.ai artificial intelligence study, 2020.

6 Hematopoietic Stem Cell Committed Lymphoid Progenitor Cell T Lymphocytes B Lymphocytes Multipotent Stem Cell Committed Myeloid Progenitor Cell Neutrophils Monocytes BONE MARROW BLOOD Maturation MobilizationExpansion Mature neutrophil pool in bone marrow is 20 times higher than in circulation1 Source: Bainton DF (1980) The cells of inflammation: a general view. In Weissmann G (ed) The Cell Biology of Inflammation, vol 2, pp 1–25. Amsterdam: Elsevier/North-Holland Neutrophils are ~50% of circulating immune cells1 CXCR4 Plays Key Role in the Expansion, Maturation & Mobilization of Immune Cells CXCR4 receptor upregulation / dysfunction can cause a range of chronic neutropenic disorders

7 Mavorixafor: Realizing the Potential of CXCR4 Antagonism in an Oral Capsule Only oral CXCR4 antagonist in development • Small molecule with high potency and selectivity • Durable half-life supporting once-daily dosing • 2 or 4 capsules, once per day (WHIM syndrome) Antagonizes Both Wild-Type and Mutated CXCR4 • Demonstrated ability to increase the mobilization of white blood cells, including neutrophils, from bone marrow to bloodstream • Potential utility across broad range of chronic neutropenic disorders with high unmet needs Safety Profile Supports Chronic Use • >200 patients/subjects treated to date • Some patients on Rx for several years Favorable Regulatory Designations (WHIM syndrome) • Breakthrough Therapy Designation (U.S.) • Fast Track Designation (U.S.) • Rare Pediatric Disease Designation (**PRV eligible) • Orphan Drug Status in U.S. and Europe Patent Protection Expected Through 2038 and Beyond

8 Mavorixafor Clinical Data: First Oral Treatment with Broad Durable Increases in Peripheral White Blood Cell Counts Across All Populations Studied Mavorixafor in WHIM SyndromeMavorixafor in Healthy Volunteers Stone et al, Antimicrobial Agents and Chemotherapy, July 2007 ASH 2021. Poster #2186. WBC: white blood cells; ANC: absolute neutrophil count; ALC: absolute lymphocyte count; AMC: absolute monocyte count

9 Pipeline: Significant Upcoming Milestones Expected Candidate Indication Preclinical Phase 1 Phase 2 Phase 3 Expected Milestones Target Patient Populations C H R O N IC N E U T R O P E N IC D IS O R D E R S (I n -h o u s e ) Mavorixafor WHIM Syndrome 1 (Warts, Hypogammaglobulinemia, Infections and Myelokathexis) Top-line data 4Q 2022 NDA Submission early 2H 2023 > 1,000 U.S.2 Chronic Neutropenia (Congenital, Cyclic, and Idiopathic) Add’l data / clinical update in 3Q 2022 > 5,000 U.S.3 X4P-003 TBD O N C O L O G Y (P a rt n e ri n g ) Mavorixafor Waldenström’s Macroglobulinemia* (double-mutation patients) > 2,000 U.S.4 X4P-002 Oncology indications* Other leukemias and lymphomas > 25,000 U.S.3 Phase 3 Phase 1b Phase 1b IND-enabling *Programs only being advanced through partnership Sources: 1. Phase 3 open label extensions (OLE) for WHIM ongoing 2. Company market research. Qessential market research, 2019 and IPM.ai artificial intelligence study, 2020. 3. Estimate using Andersen et al. J Intern Med. 2016 Jun;279(6):566-75. 4. WM Epidemiology Analysis The NemetzGroup.

10 WHIM Syndrome: an Immunodeficiency Affecting Children and Adults Leanne’s Story - Living with WHIM Syndrome Pancytopenia: All White Blood Cells Affected & Reduced Severe bacterial infections In multiple organ systems: bronchiectasis (lung), hearing loss (ear), cellulitis (skin) Viral infections & cancer risk Disfiguring recalcitrant warts; EBV and HPV- associated cancers WHIM Patient New South Wales, Australia 0 Approved targeted therapies; symptomatic treatment with G-CSF and IVIG www.whimsyndrome.com [In early marriage], I had 8 episodes of pneumonia in 8 months, more likely one continual episode of pneumonia. I had a PICC line, I was having intravenous antibiotics. Every time I was better, they’d stop the antibiotics and almost immediately I’d become unwell again.

11 Root Cause of WHIM Syndrome: Over-Signaling of CXCR4 CXCR4 Receptor Mutations cause over-signaling Impacting immune cell expansion, maturation, and mobilization • WHIM Syndrome: Spectrum of clinical presentations mostly with (and sometimes without) CXCR4 mutations • W: warts • H: hypogammaglobulinemia • I: infections • M: myelokathexis (hyper-cellular bone marrow) • In most cases, autosomal dominant disease driven by pathogenic CXCR4 mutations; mostly located in "tail" (c-terminus) of receptor • ~16 identified to date; # increasing with research • Results in over-signaling of CXCR4 that impacts all white blood cells D

12 EHA 2020: Phase 2 WHIM Trial Demonstrates Activity Across Phase 3 Endpoints Major (>600%) Increase in Neutrophil Counts (TATANC) >80% Reduction in Annualized Infection Rates WHIM Phase 2 Trial Design • Intra-patient dose- escalation: safety, PK • Endpoint: clinically relevant blood measurement: "Time above threshold for absolute neutrophil count" (TATANC) • FDA & EMA agreement on use as P3 endpoint • Wart burden and infection rates also examined • Patients eligible to participate in an open-label extension (OLE) phase of the trial >75% reduction in the number of warts while on treatment 0 2 4 6 8 10 12 14 50-200 mg 300 mg 400 mg M e a n T A T A N C (h o u rs )
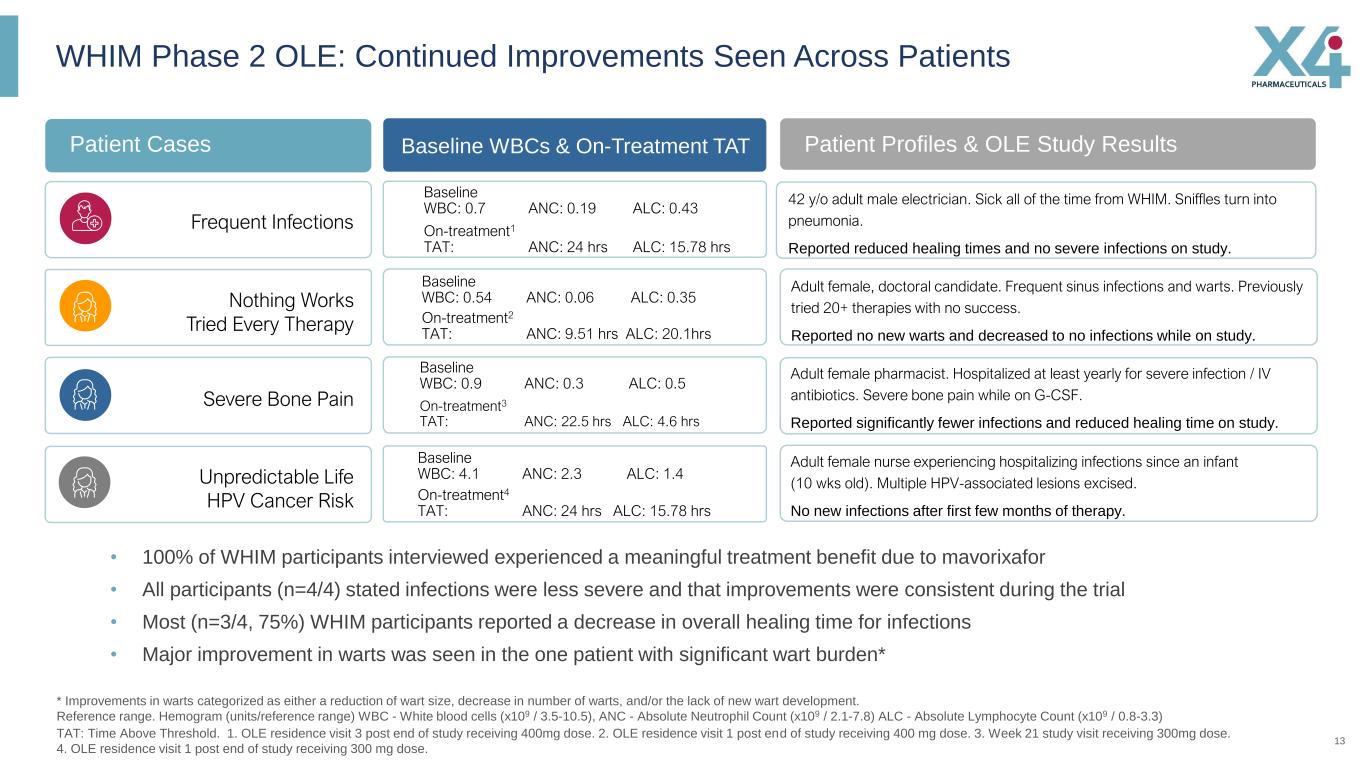
13 WHIM Phase 2 OLE: Continued Improvements Seen Across Patients * Improvements in warts categorized as either a reduction of wart size, decrease in number of warts, and/or the lack of new wart development. Reference range. Hemogram (units/reference range) WBC - White blood cells (x109 / 3.5-10.5), ANC - Absolute Neutrophil Count (x109 / 2.1-7.8) ALC - Absolute Lymphocyte Count (x109 / 0.8-3.3) TAT: Time Above Threshold. 1. OLE residence visit 3 post end of study receiving 400mg dose. 2. OLE residence visit 1 post end of study receiving 400 mg dose. 3. Week 21 study visit receiving 300mg dose. 4. OLE residence visit 1 post end of study receiving 300 mg dose. • 100% of WHIM participants interviewed experienced a meaningful treatment benefit due to mavorixafor • All participants (n=4/4) stated infections were less severe and that improvements were consistent during the trial • Most (n=3/4, 75%) WHIM participants reported a decrease in overall healing time for infections • Major improvement in warts was seen in the one patient with significant wart burden* Patient Cases Baseline WBCs & On-Treatment TAT Baseline WBC: 0.7 ANC: 0.19 ALC: 0.43 Baseline WBC: 0.54 ANC: 0.06 ALC: 0.35 Baseline WBC: 0.9 ANC: 0.3 ALC: 0.5 Baseline WBC: 4.1 ANC: 2.3 ALC: 1.4 Patient Profiles & OLE Study Results 42 y/o adult male electrician. Sick all of the time from WHIM. Sniffles turn into pneumonia. Reported reduced healing times and no severe infections on study. Adult female, doctoral candidate. Frequent sinus infections and warts. Previously tried 20+ therapies with no success. Reported no new warts and decreased to no infections while on study. Adult female pharmacist. Hospitalized at least yearly for severe infection / IV antibiotics. Severe bone pain while on G-CSF. Reported significantly fewer infections and reduced healing time on study. Adult female nurse experiencing hospitalizing infections since an infant (10 wks old). Multiple HPV-associated lesions excised. No new infections after first few months of therapy. Frequent Infections Severe Bone Pain Unpredictable Life HPV Cancer Risk On-treatment1 TAT: ANC: 24 hrs ALC: 15.78 hrs On-treatment2 TAT: ANC: 9.51 hrs ALC: 20.1hrs On-treatment3 TAT: ANC: 22.5 hrs ALC: 4.6 hrs On-treatment4 TAT: ANC: 24 hrs ALC: 15.78 hrs Nothing Works Tried Every Therapy

14 Design of Mavorixafor Global Phase 3 Trial in WHIM (Patients ≥12 years of age) • Primary Endpoint: Biomarker of time above threshold (>500 cells per microliter) for absolute neutrophil count (TATANC); average of four assessment timepoints • Secondary Endpoints: TATALC, infections, wart burden, infection and wart composite, QoL assessment & others • Dosing: 400mg QD in patients for subjects above 50 kg; 200 mg QD for those below 50 kg and <18 years • Enrollment Complete: Over-enrolled with 31 patients Establish TAT Baseline 1 : 1 Randomization 3 mos. 6 mos. 9 mos. 12 mos. Mavorixafor (N=9-14) Placebo (N=9-14) Rollover to Open-label Trial Primary Endpoint Assessed Phase 3 Top-line Data Expected in 4Q 2022

15 1. Pathogenic / likely pathogenic CXCR4 mutations – including variants causing gain-of-function CXCR4 signaling 2. Myelokathexis Understanding the Definition and Diagnosis of WHIM Syndrome For the treatment of WHIM Syndrome in patients ≥12 years Diagnosis of WHIM Syndrome: Clinical and Laboratory Presentation* consistent with WHIM (≥ 1 of the following: warts, lymphopenia, hypogammaglobulinemia, neutropenia, and/or infections) Targeted Mavorixafor U.S label The Different Faces of WHIM Diagnostic paradigm is evolving; some leading clinicians would consider diagnosing WHIM without CXCR4 or myelokathexis (which can easily be missed)

16 Chronic Neutropenia Beyond WHIM Chronically immunocompromised patients due to sustained or intermittent low neutrophil counts (neutropenia); can be caused by • G-CSF response defects • Elevated bone marrow CXCL12 downregulating G-CSF production • Stem cell loss • Differentiation/maturation arrest • Increased apoptosis • Prolonged survival of senescent neutrophils preventing regeneration of nascent neutrophils • Impaired release from bone marrow • Impaired de-margination from periphery • Functional upregulation of CXCL12/CXCR4 pathway The magnitude of neutropenia correlates with higher risk of severe infections and greater frequencies of infections • Mild if ANC between 1,000 and 1,500/µL • Moderate if ANC between 500 and 1,000/µL • Severe if ANC <500/µL (estimated 5,000 U.S. patients1,2) 1. Eligible patients in the TriNetX USA database who had an ANC <1500 cells/μl in calendar year of interest and two additional ANC <1500 cells/μl in the subsequent two years. 2. Numbers are extrapolated to reflect the total U.S. population each year (multipliers ranged from x6.4 in 2017 to x5.2 in 2019). Pneumonia Oral Ulcerations Skin Abscesses

17 Chronic Neutropenia Management: Currently Approved Treatments Have Significant Limitations and High Side-Effect Burden Injections of G-CSF • Once or twice-daily at 5-6 mg/kg to reduce severe neutropenia and risk of infections • “Dose down-titration” and reduced frequency often implemented to aid with tolerability for chronic use • Neutrophil target: ~1,000-2,000 cells/µL Challenges for patients on G-CSF • 25% continue to experience severe bacterial infections while on chronic treatment1 • Increasing risk of myelodysplastic syndromes (MDS)2 • ~70% have moderate or severe bone-pain impacting compliance and QoL3 No alternative therapies, except for bone marrow transplantation 1. Fontbruen et al, Blood, October 2015 – Volume 128(14). 2. Dale et al Support Cancer Ther 2006 Jul 1;3(4):220-31:. 3. Michniacki et al, Blood (2019) 134 (Supplement_1): 3449. U.S. G-CSF Market For Severe Chronic Neutropenia: Estimated $120-460 Million

18 > 20,000 People (aged >12) Estimated with Chronic Neutropenia in the U.S.1: Thousands Have Major Health Impact with >2 Serious Infection Events per Year 1. Eligible patients in the TriNetX USA database who had an ANC <1500 cells/μl in calendar year of interest and two additional ANC <1500 cells/μl in the subsequent two years. 2. Numbers are extrapolated to reflect the total U.S. population each year (multipliers ranged from x6.4 in 2017 to x5.2 in 2019). 4,986 5,677 5,340 0 5,000 10,000 15,000 20,000 25,000 2017 2018 2019N u m b e r o f P e o p le w it h C h ro n ic N e u tr o p e n ia † >2 Serious Infection Events All Chronic Neutropenia 18,400 19,443 19,048 ~25% of all CN patients have two or more SIEs 2 per year Source: X4 Data on file; TriNetX USA EMR Database analysis using inclusion criteria and medical record coding, data and confirmation. Patients 12 years of age and above. CN Patients Experience Serious Infection Events (SIEs): Any infection that required hospitalization, intravenous antibiotics and/or resulted in disability or death

19 Protocol Version 5 • 14 days of treatment with mavorixafor • Blood sampling over 6 hours on days -1, 1 and 14 • Severe (ANC<500/uL) chronic idiopathic neutropenias on concomitant G-CSF • Selected severe congenital neutropenia conditions with or without concomitant G-CSF • Clinic only study Protocol version 6 • 1 day of treatment • Blood sampling over 8 hours on days -1 and 1 • Any congenital, cyclic, or idiopathic neutropenic disorder regardless of concomitant G-CSF use • Neutrophil count <1,000 µL if not on G-CSF • Study can be done in the clinic or at home Phase 1b trial: Studies Activity of Mavorixafor in Broad CN Population • Severe and moderate CN – congenital, idiopathic, cyclic • With or without genetic causes • With or without concomitant G-CSF Endpoints: Safety and tolerability, change in ANC (and other WBCs) vs. pre-treatment baseline Goal: Define broad CN populations responsive to mavorixafor in support of FDA interactions regarding proposed registrational trial(s) Studying mavorixafor across broad chronic neutropenic disorders Fully Enrolled Phase 1b Trial: Assessing Mavorixafor in Patients with CN Patients 12 years old and older

20 ASH 2021: Mavorixafor Impact on WBCs in Chronic Neutropenia Patients Meaningful Responses* to Single Dose in Idiopathic Chronic Neutropenia Patients Absolute Neutrophil Count (ANC) * ASH 2021. Poster #2186. >2-fold increase in ANC after single dose of mavorixafor Similar increases in: ✓ Total White Blood Cell Counts (WBCs) ✓ Absolute Lymphocyte Counts (ALC) ✓ Absolute Monocyte Counts (AMC)

21 Significant Opportunity for Mavorixafor in Chronic Neutropenia ✓More than 5,000 patients in the U.S. with chronic neutropenia at significantly increased rates of hospitalization with severe infections ✓Only available treatment is injectable, with limited tolerability and frequent injections ✓Mavorixafor single dose activity: Initial data from the ongoing Phase 1b trial in adult patients with idiopathic CN show clear elevation of neutrophils and other WBCs ✓ Further positive data from this ongoing trial to inform registrational trial to investigate mavorixafor as the potential first oral treatment to reduce the burden of infections in patients with chronic neutropenia Fully enrolled Phase 1b trial; Readout expected late September 2022

22 Building to Support a Continuum of Patients with Chronic Neutropenic Disorders " Classic WHIM WHIM Expansion: VUS, Myelokathexis, and Other Chronic Neutropenia Efficiently leveraging resources and time across programs

23 Recent and Key Upcoming Expected Milestones Recent Events: • Raised gross proceeds of ~$56 million from PIPE financing in June 2022 • Amended debt facility with Hercules Capital extending interest-only payment period into 2024 • Announced sharpening of corporate focus and resources on development of mavorixafor for chronic neutropenic disorders, including WHIM syndrome • Updated X4 oncology programs • Positive results from Phase 1b data of mavorixafor for the treatment of Waldenström’s Macroglobulinemia (WM) • Announced granting of U.S. Orphan Drug Designation for mavorixafor in WM regardless of CXCR4 mutation status • Advancement of mavorixafor WM and pre-clinical X4P-002 programs only to proceed upon strategic partnership(s) Upcoming Expected Milestones • Late September 2022 presentation of data from fully enrolled Phase 1b trial of mavorixafor in chronic neutropenia patients • 4Q 2022 unveiling of data from global, pivotal Phase 3 (4WHIM) clinical trial of mavorixafor for the treatment of patients with WHIM syndrome • Early 2H 2023 submission for U.S. regulatory approval of mavorixafor for WHIM • Clarification of regulatory path forward for mavorixafor in CN indications

24 Strong Balance Sheet Supports Expected Upcoming Milestones 1 Pro-forma for June 30, 2022 cash and restricted cash balance of $48.7 plus estimated net proceeds from PIPE financing that closed on July 6, 2022. 2 As described in detail in our most recent Form 10-Q, our agreement with Hercules Capital, Inc. contains a minimum cash covenant that becomes effective on September 1, 2022, subject to certain exceptions. Our current cash runway projections assume continued compliance with this covenant. Failure to satisfy this covenant could result in accelerated principal and interest payments due that could shorten our cash runway. Outstanding debt balance as of 6/30/2022 is $32.5 million. Analyst Coverage Cash Expected to Fund Operations into 3Q 20232 Top-tier Life Science-Focused Institutional Shareholder Base $101M1 (pro-forma including recent PIPE financing)

25 U.S. Headquarters 61 North Beacon Street, 4th Floor Boston, MA 02134 Research Facilities Helmut-Qualtinger-Gasse 2 A-1030 Vienna, Austria www.x4pharma.com NASDAQ: XFOR
